Introduction
Drug provocation test timing plays a critical role in evaluating suspected drug allergies. Drug provocation testing (DPT), also known as a drug challenge, remains the gold standard for confirming or ruling out drug hypersensitivity. However, success depends heavily on understanding how soon after drug exposure symptoms appear. By tailoring the timing and protocol of DPT based on the type of reaction—immediate, delayed, or severe—clinicians can improve diagnostic accuracy and minimize risk. This post outlines practical strategies, guideline updates, and real-world challenges that affect how and when drug provocation tests should be performed.
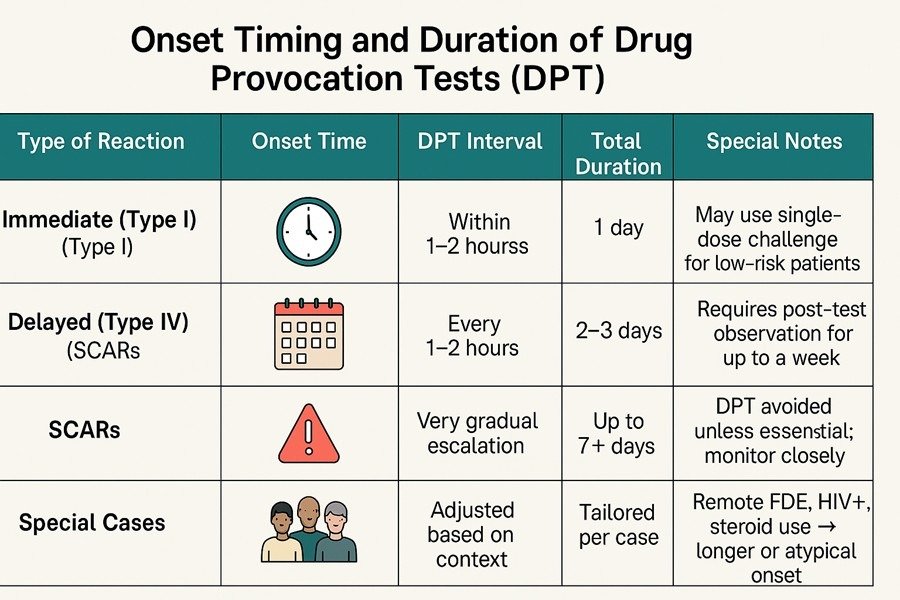
1. Immediate Hypersensitivity Reactions (Type I)
- Onset: Minutes to 1–2 hours
- DPT Interval: Every 30–60 minutes
- Total Duration: Usually completed in one day
- Notes: Safe for low-risk patients. A single full-dose challenge is often sufficient. Supported by EAACI (2024) and AAAAI (2022).
2. Non-Immediate Hypersensitivity Reactions (Type IV)
- Onset: 4–14 days after exposure
- DPT Interval: Every 1–2 hours
- Total Duration: 2–3 days
- Notes: Observe for up to 7 days after completion. Common in maculopapular exanthema (MPE).
Update: Both EAACI and AAAAI suggest that a short 1–3 day DPT may suffice for low-risk delayed reactions.
3. Severe Cutaneous Adverse Reactions (SCARs)
Includes: DRESS, SJS/TEN, AGEP
- Onset: 7–21 days (can be up to 2 months)
- DPT Interval: Very gradual if performed at all
- Total Duration: Up to 7+ days with close monitoring
- Notes: Generally contraindicated with the culprit drug; may be considered for alternatives if essential.
4. Drug-Specific Strategies
- Beta-Lactams: Direct single-dose DPT is acceptable in low-risk cases. Supported by both EAACI and AAAAI.
- NSAIDs: Use phenotype-based stratification; trials to confirm tolerance.
- Sulfonamides, Macrolides, Fluoroquinolones: Skin testing has low value; DPT is preferred for non-anaphylactic reactions.
5. Special Clinical Situations
Keep in mind that the information above serves only as a general guideline. In certain circumstances, we have encountered cases that did not follow these standard rules. For example:
- Fixed Drug Eruption (FDE) Reactivation Beyond Day 3: A patient with a history of levofloxacin-induced FDE 10 years prior developed a recurrence on Day 4, despite negative patch testing.
- Delayed Immune Recovery in HIV: An HIV-infected woman with a remote history of sulfa allergy and a low CD4 count developed MPE/DRESS after 7 days of cotrimoxazole rechallenge, despite an initially negative ELISpot. A repeat ELISpot later turned positive following the onset of the reaction.
- DRESS After Intermittent Dosing + Steroids: Another patient developed DRESS 3 months after starting cotrimoxazole, which was prescribed twice weekly in combination with high-dose corticosteroids.
These examples illustrate that immunodeficiency, intermittent drug exposure, and a remote history of allergy may lead to delayed-onset reactions that fall outside standard DPT timeframes.
6. What If You Don’t Know the Reaction Type?
In real-world practice, patients may not recall the timing of their previous drug reaction, and not all reactions fit neatly into type I or IV hypersensitivity. Sometimes, it is essential to use the suspected drug—even when the reaction was not clearly mild—yet the type of hypersensitivity remains inconclusive.
Other mechanisms include:
- Type II: Drug-induced cytopenias
- Type III: Serum sickness, vasculitis
- Other immune reactions: drug-induced liver injury, drug-induced autoimmune hepatitis, cytokine release syndrome, complement activation-related pseudoallergy
Suggested approach:
- Perform both immediate and delayed skin tests
- Utilize ELISpot or LTT if available
- If all negative, consider a carefully monitored DPT using non-immediate intervals with extended observation
References
- Khan DA, et al. Drug allergy: a 2022 practice parameter update. J Allergy Clin Immunol. 2022;150(6):1333–1343.
- Barbaud A, et al. EAACI/ENDA position paper on drug provocation testing. Allergy. 2024;79(3):565–579.
- Brockow K, et al. Skin test concentrations for systemically administered drugs. Allergy. 2013;68(6):702–12.
- Romano A, et al. Towards a more precise diagnosis of hypersensitivity to beta-lactams. J Allergy Clin Immunol Pract. 2020;8(10):3321–8.
- Labella M, et al. Direct single-dose DPT is safe for delabelling penicillin low-risk reactions. Allergy. 2025;0:1–13.
- Our clinical observations and unpublished case reports.